Find the Percent Composition of Each Element in Caffeine C8h10n4o2
5 rows Element. Chemical Calculations - The Mole Concept Molar mass of caffeine C8H10N4O2 _____gmole 651 moles of citronellal a natural insect repellent C10H18O _____ g.

Solved 5 Find The Percent Composition Of Each Element In Chegg Com
Carbon 4948 Determine the percent composition of caffeine.

. Atomic Mass of Atoms. C 25 C 25 H 25 N 25 N and 25 O. H31008 g H 9799 g H3PO4100 3086 P3097 g P 9799 g H3PO4100 3161 O41600 gO 9799 g H3PO41006531 Check Do the percentages add to 100 percent.
Arrow_forward The average nicotine C 10H 14N 2 molar mass 1623 gmol content of a Camel cigarette is 193 mg. Carbon 4948 hydrogen 519 oxygen 1648 and nitrogen 2885. Caffeine has the molecular formula C8H10N4O2.
Now click the button Calculate Percent Composition to get the result. 4 rows Elemental composition of C8H10N4O2caffeine Element Symbol Atomic weight Atoms Mass. Use three significant figures in your answers.
B 4948 C 519 H 2885 N and 1648 O. Use three significant figures in your answers. M H 1 gmol x 10 10.
Find the percent composition of each element in caffeine C8H10N4O2. D 2792 C 234 H 3256 N and 3719 O. 4 mol N 560268 g.
Percent composition indicates the relative amounts of each element in a compound. Then find the ratio of the elements to the smallest one with it being no less than 100. Calculate the mass percent composition of each element in the explosive TNT C 6 H 2 NO 2 3 CH 3.
See the answer Show transcribed image text Expert Answer 100 1 rating Molar mass of caffeine C8H10N4O2 8 molar mass of carbon 10 molar mass of hydrogen 4 molar mass of nitrogen 2 molar. M C 12 gmol x 8 96. Mass mass of element in 1 mole of the compound molar mass of the compound x 100.
Al 3462 O 6154 H 385 The Percentage Composition by Mass of Each Element is indicated by - Molar mass of The ElementMolar Weight of the Total Compound xx 100 So The Molar Weight of the Total Compound 27 3 xx 16 1 g mol-1 27 51 g mol-1 78 g mol-1 So Percentage Composition of Aluminium 2778 xx 100 3462. A Calculate the molar mass of caffeine. Then the by weight of each element would be.
Molar mass of caffeine C8H10N4O2 812110414216. What is the percent composition of caffeine. From the previous problem we know that 8 mol C 96088 g.
Lets work another example with our favorite molecule caffeine. The easy way to do that is to divide the smallest number by itself thereby assuring a 100 for that element then divide all of the other numbers by the same small number round to whole numbers and that should be the empirical formula. 19419 Determine the percent composition of caffeine.
Its molecular weight is. 960881941926 100 4948. Finally the chemical formulas percent composition will be displayed in the new window.
Calculating the molecular formula of caffeine. At first find the molar mass of each element in caffeine. The percent by mass of each of the elements in H3PO4 is calculated as follows.
For each element the mass percent formula is. Calculate the mass percent composition of each element in the explosive TNT C6H2NO23CH3. Caffeine is a methylxanthine alkaloid found in the seeds nuts or leaves of a number of plants native to South America and East Asia that is structurally related to adenosine and acts primarily as an adenosine receptor antagonist with psychotropic and anti-inflammatory activities.
Suppose an individual smokes one pack of 20 cigarettes a day. Caffeine has the molecular formula C8H10N4O2. The chemical formula of caffeine is C8H10N4O2 and the molecular mass is 19419.
Enter the chemical formula in the input field. Element Symbol Atomic Mass Number of Atoms Mass Percent. What is the percent composition of caffeine.
2 mol O 319988 g. Nitrogen 29 Determine the percent composition of caffeine. What element has the highest percentage by mass in hydrated penta-aqua copper II sulphate.
A 3333 C 4167 H 1667 N and 833 O. A 3333 C 4167 H 1667 N and 833 O. Chapter 3 I Chemical Formulas a How many atoms of each type of element are present in Cr.
For a molar mass of 1941926 gmol. It has a molar mass of 1942 gmol -1. Caffeine has the following percent composition.
Caffeine contains 4948 Carbon 515 Hydrogen 2887 nitrogen 1649 oxygen. 10 mol H 10079 g. 100791941926 100 519.
Hydrogen 519 Determine the percent composition of caffeine. View the full answer Transcribed image text. Solution The molar mass of H3PO4 is 9799 g.
- Carbon - 49464 - Nitrogen - 28860 - Oxygen - 16483 - Hydrogen - 5193 Home Subjects Math Science. The stimulant effect of coffee is due to caffeine C8H10N4O2. Find the percent composition of each element in caffeine C3H1N02.
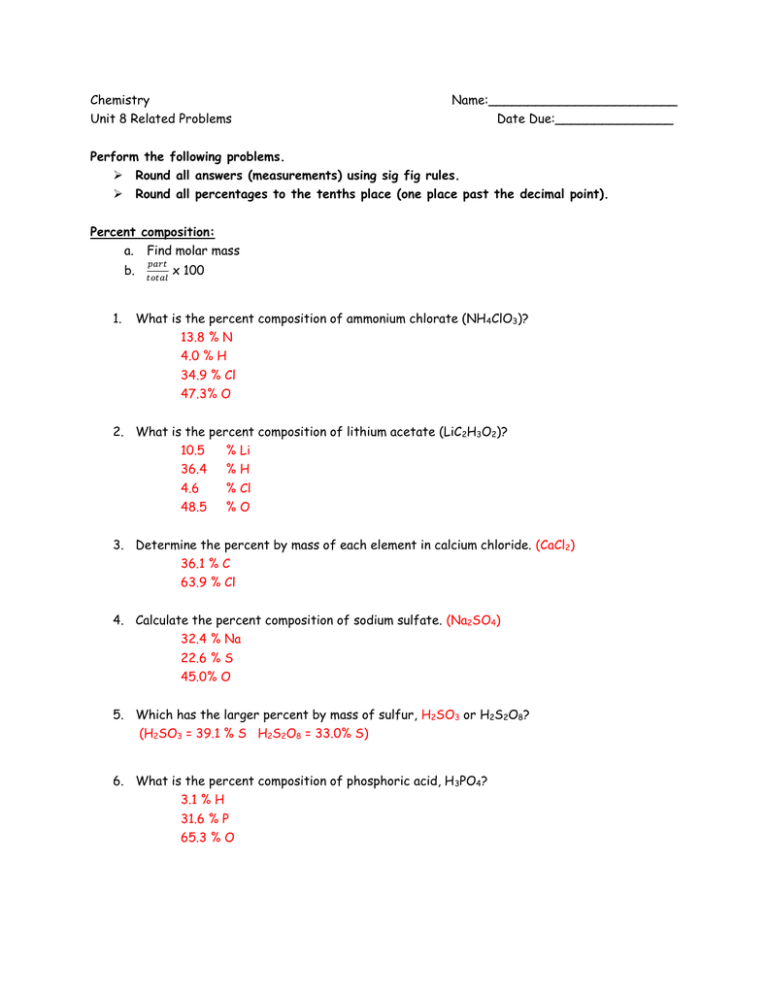
Chemistry Name Unit 8 Related Problems Date Due Perform The

Empirical Molecular Formula Of Caffeine From Composition Data Youtube

Oneclass Caffeine Is A Bitter Stimulant Drug And Is Found In Varying Quantities In Seeds Leaves An
Caffeine Has A Molecular Weight Of 194 If It Contains 28 9 By Mass Of Nitrogen What Is The Number Of Atoms Of Nitrogen In One Molecule Of Caffeine Quora

Composition Of Substances And Solutions Ppt Download
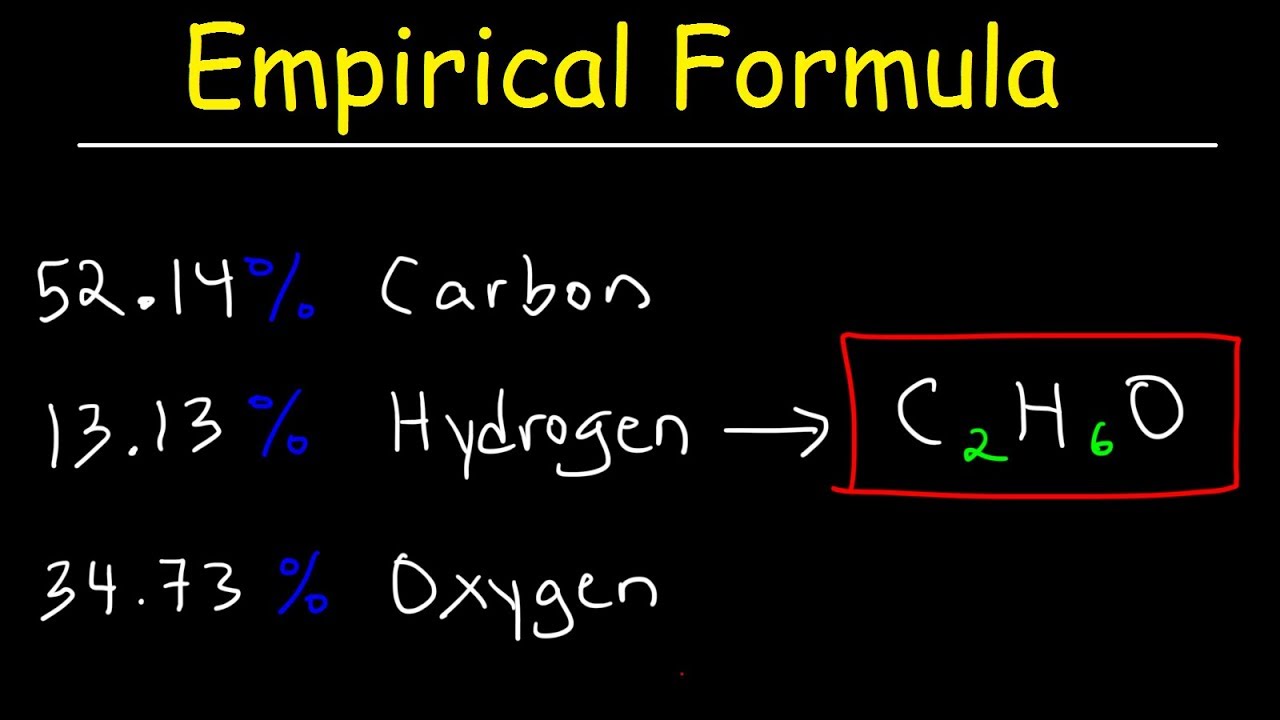
What Is The Empirical Formula For C4h10o2 Lisbdnet Com

Aim How To Calculate Percent Composition Ppt Download

Composition Of Substances And Solutions Ppt Download

What Is The Molecular Formula Of Fructose If Its Molar Mass Is 180 G Mol And Its Empirical Formula Is Ch2o

Find The Empirical Formula Of Caffeine C8h10n4o2 Youtube

Question Video Formula Mass And Mole Concept Calculation Of Molar Mass Nagwa

Percent Composition Review Ppt Download
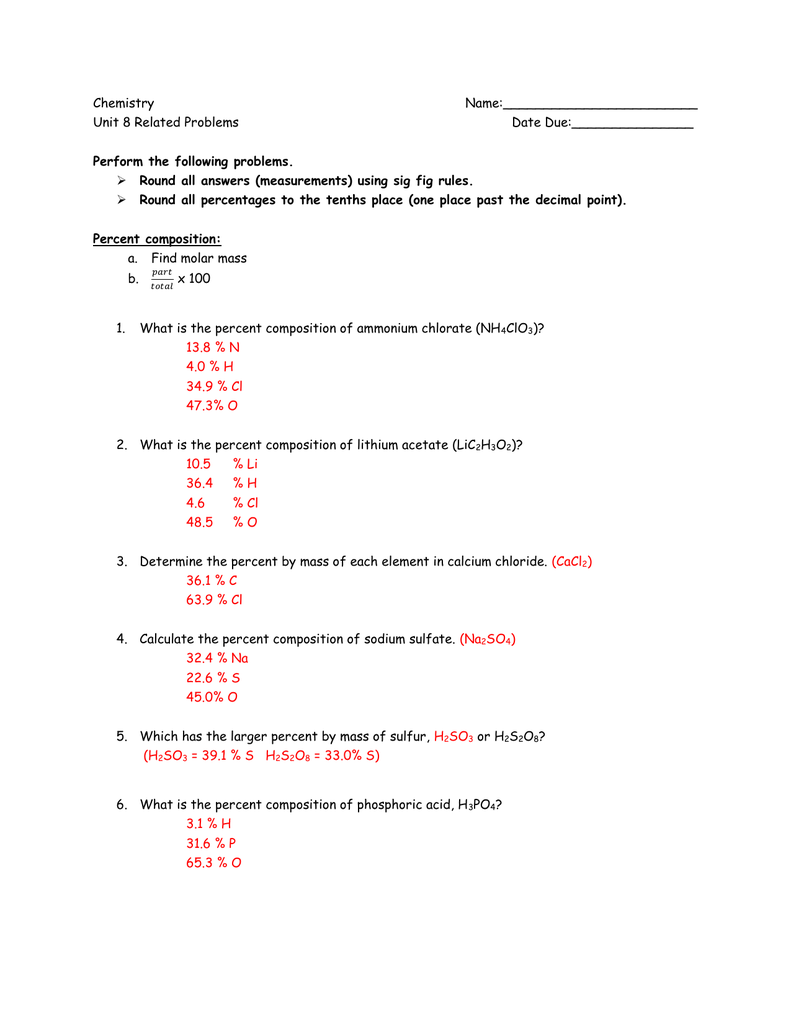
Chemistry Name Unit 8 Related Problems Date Due Perform The

Solved 1 Calculate The Percent Composition Of Each Element Chegg Com

Empirical Molecular Formula Of Caffeine From Composition Data Youtube

How To Calculate The Molar Mass Of C8h10n4o2 Caffeine Youtube

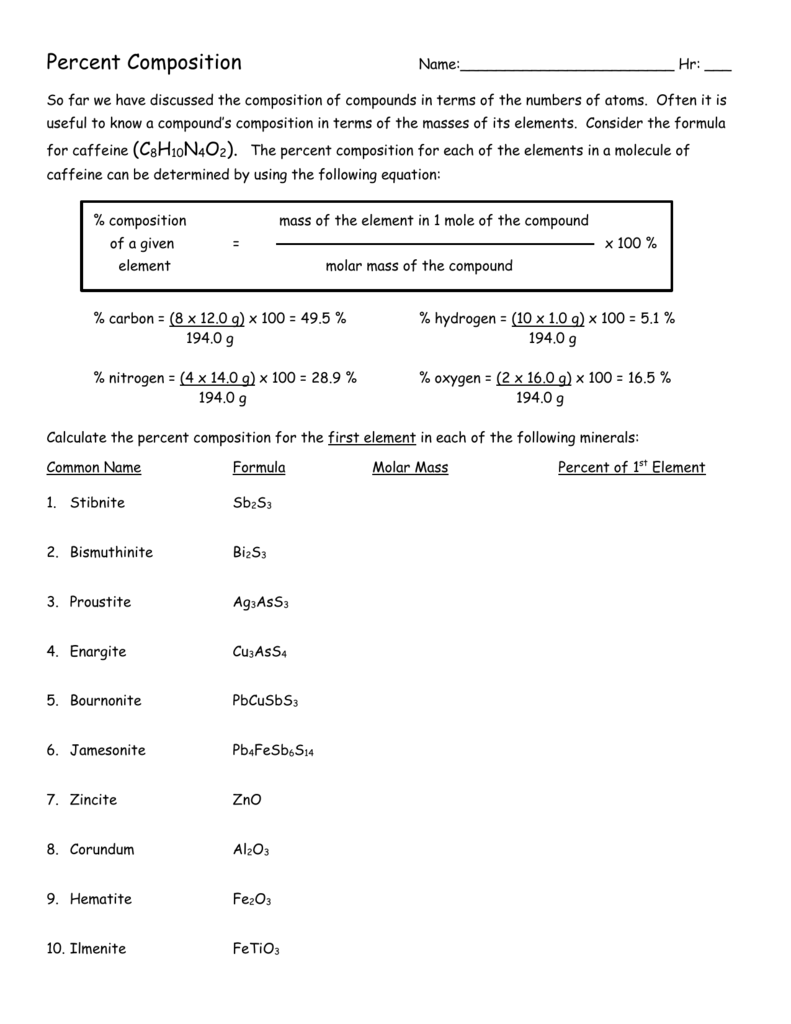

Comments
Post a Comment